is carbon monoxide polar Vsepr monoxide nonpolar
Carbon monoxide (CO) is a highly significant compound that has various applications in different industries. Understanding its polarity and whether it is ionic or covalent is essential for comprehending its chemical properties and reactivity.
Is CO Polar or Nonpolar?
When examining the structure of carbon monoxide (CO), we can determine whether it is a polar or nonpolar molecule. The polarity of a molecule is influenced by the electronegativity difference between the atoms and the molecular shape.
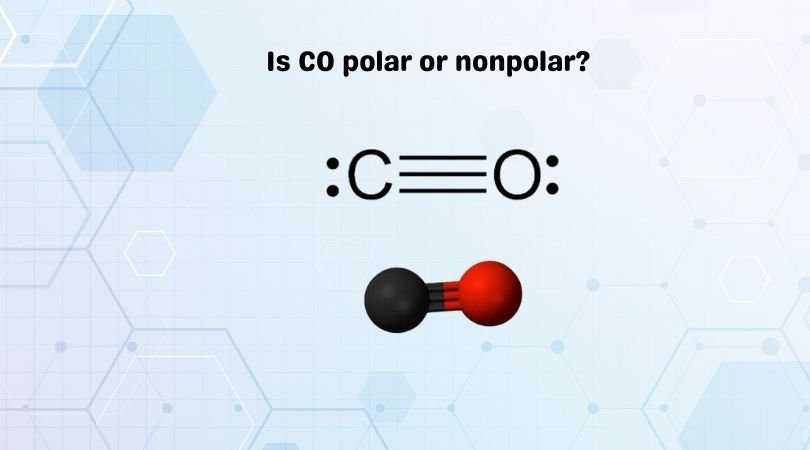 In the case of carbon monoxide, the carbon-oxygen bond is polar due to the difference in electronegativity between the two atoms. Oxygen is more electronegative than carbon, causing it to exert a stronger pull on the shared electrons. As a result, oxygen becomes partially negative (δ-) while carbon becomes partially positive (δ+).
In the case of carbon monoxide, the carbon-oxygen bond is polar due to the difference in electronegativity between the two atoms. Oxygen is more electronegative than carbon, causing it to exert a stronger pull on the shared electrons. As a result, oxygen becomes partially negative (δ-) while carbon becomes partially positive (δ+).
The molecular shape of carbon monoxide is linear, with the carbon atom in the center and the oxygen atom on one side. This linear arrangement further contributes to the polarity of CO. The polar bond combined with the linear shape leads to an overall polarity in the molecule.
Is CO Ionic or Covalent?
Unlike ionic compounds that consist of positive and negative ions held together by electrostatic forces, carbon monoxide is a covalent compound. Covalent compounds are formed when two or more nonmetals share electrons to complete their valence shells.
 Carbon and oxygen, both nonmetals, form a covalent bond in CO by sharing a pair of electrons between them. This sharing of electrons allows both atoms to achieve stability by filling their outer electron shells, resulting in a stable molecule.
Carbon and oxygen, both nonmetals, form a covalent bond in CO by sharing a pair of electrons between them. This sharing of electrons allows both atoms to achieve stability by filling their outer electron shells, resulting in a stable molecule.
In summary, Carbon monoxide (CO) is a polar covalent molecule. It has a polar bond due to the difference in electronegativity between carbon and oxygen, and its linear molecular shape enhances its overall polarity.
Understanding the polarity and bonding nature of carbon monoxide is crucial in various fields. In the chemical industry, carbon monoxide is utilized in the synthesis of a wide range of important organic compounds. Additionally, it plays a vital role in the production of methanol, acetic acid, and other essential chemicals.
Moreover, carbon monoxide is present in the atmosphere, mainly produced by the incomplete combustion of fossil fuels. It is a significant air pollutant, and its exact understanding is crucial for environmental scientists to develop effective strategies for reducing its emissions.
In conclusion, carbon monoxide (CO) is a polar covalent molecule with a linear molecular shape. Its polarity arises from the polar carbon-oxygen bond, making it a crucial compound in various industrial processes and environmental studies.
If you are looking for Is CO (Carbon Monoxide) polar or nonpolar? : Check Polarity you’ve came to the right place. We have 5 Images about Is CO (Carbon Monoxide) polar or nonpolar? : Check Polarity like Is CO (Carbon Monoxide) polar or nonpolar? : Check Polarity, Polar HVAC NM | Carbon monoxide Poisoning in Albuquerque NM and also Is CO (Carbon Monoxide) polar or nonpolar? : Check Polarity. Here you go:
Is CO (Carbon Monoxide) Polar Or Nonpolar? : Check Polarity
 geometryofmolecules.comnonpolar polarity monoxide
geometryofmolecules.comnonpolar polarity monoxide
Polar HVAC NM | Carbon Monoxide Poisoning In Albuquerque NM
 polarhvacnewmexico.commonoxide carbon poisoning prevent guidelines following simple some nm
polarhvacnewmexico.commonoxide carbon poisoning prevent guidelines following simple some nm
Is CO Ionic Or Covalent? - Techiescientist
 techiescientist.commonoxide carbono monoxido covalent karbon ikatan monoksida carbone kovalen molecule monóxido nonpolar estructural oxide dative bonds chem
techiescientist.commonoxide carbono monoxido covalent karbon ikatan monoksida carbone kovalen molecule monóxido nonpolar estructural oxide dative bonds chem
Carbon Monoxide Polar Or Nonpolar - Google Search | Molecular Shapes
 www.pinterest.comvsepr monoxide nonpolar
www.pinterest.comvsepr monoxide nonpolar
Carbon Monoxide | Psychology Wiki | Fandom
 psychology.wikia.orgmonoxide carbon psychology resonance represented dipole structures moment three
psychology.wikia.orgmonoxide carbon psychology resonance represented dipole structures moment three
Monoxide carbono monoxido covalent karbon ikatan monoksida carbone kovalen molecule monóxido nonpolar estructural oxide dative bonds chem. Polar hvac nm. Is co (carbon monoxide) polar or nonpolar? : check polarity